Abstract
Acute Myeloid Leukemia (AML) is a clonal disease sprouting from a rare population of leukemic stem cells (LSCs). Over the past years, increasing interest is gaining the contribution that cell-extrinsic factors have in AML generation and maintenance. In this context, AML cell ability to sense the changes in the microenvironment is important for different cell functions, including the responsiveness to antineoplastic agents. Bitter taste receptors (T2Rs) are typical G-protein coupled receptors and are normally found on the surface of the tongue. Recent studies showed that T2Rs are widely expressed in various parts of human anatomy and have been shown to be involved in physiology of respiratory system, gastrointestinal tract and endocrine system thus suggesting a wider function in "sensing microenvironment". So far, very few data about T2Rs expression in cancer cells are available. In particular, their expression and function in AML cells has not been investigated. Our aim was to investigate T2Rs expression and role in regulation of leukemic cell functionality.
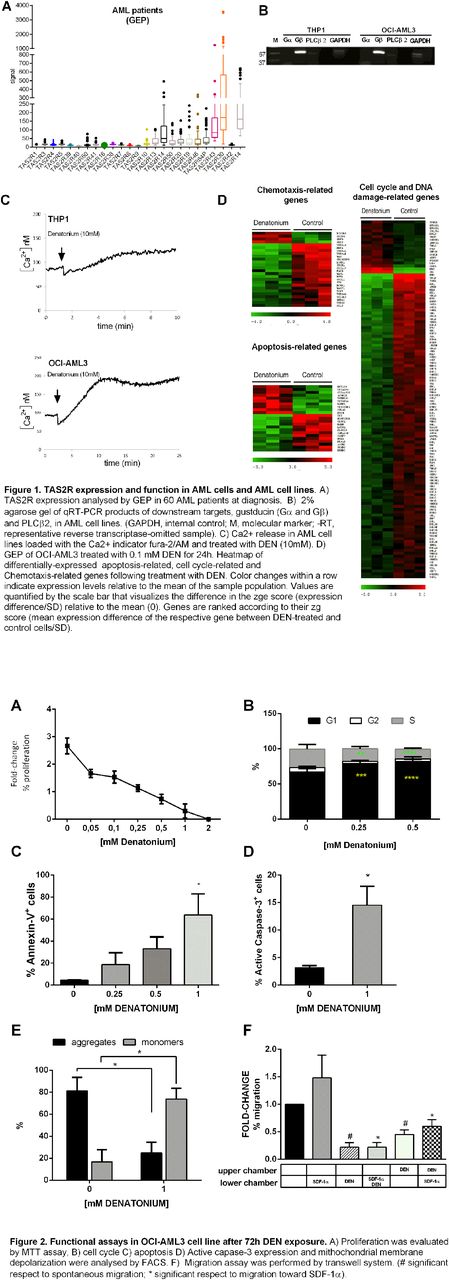
In the present work, for the first time, we showed that leukemia cell lines OCI-AML3, THP-1, KG1 and AML primary cells expressed several T2R subtypes (Fig.1A). The expression of TAS2R was associated with GPR-related downstream targets, including the beta subunit of gustducin and the PLC beta 2 (Fig.2B). Stimulation of leukemia cell lines with denatonium (DEN), a T2Rs agonist, induced intracellular Ca2+ concentration increase, thus demonstrating T2Rs functionality (Fig. 1C). To investigate whether bitter compound could modulate AML cell biological properties, we performed GEP analysis of DEN treated cells. GEP analysis identified a number of genes significantly modulated by denatonium treatment. Enrichment analysis identify significantly affected biological processes, among which, cell cycle/proliferation, apoptosis, migration/adhesion and DNA repair (Fig.1D). Specifically, leukemic cells stimulated with T2R agonist underwent down-regulation of genes involved in positive regulation of cell proliferation, migration, and cell-cycle. Whereas genes involved in cell adhesion and DNA repair were up-regulated. Functional assays confirmed molecular data. Indeed, depending on the extent of stimulation, TAS2R activation inhibited leukemia cell proliferation (Fig.2A) inducing cell cycle arrest in G0/G1 phase (Fig. 2B) or reduced cell viability inducing apoptosis (Fig. 2C), as demonstrated by caspase cascade activation and mitochondrial stress induction (Fig. 2D-E). Of note, a pronounced inhibitory effect of denatonium on leukemia cells motility, both spontaneous and in response to CXCL-12, was observed (Fig. 2F).
Overall, our results indicate that in AML cells the activation of fully functional T2Rs is associated with quiescence induction and prevention of migration. These data may suggest a role for microenvironment "bitter" molecules in regulating leukemia cell functions, including lethal sensitivity to antineoplastic agents.
Martinelli: Pfizer: Consultancy; Celgene: Consultancy; Ariad/Incyte: Consultancy; Amgen: Consultancy; Johnson&Johnson: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.